
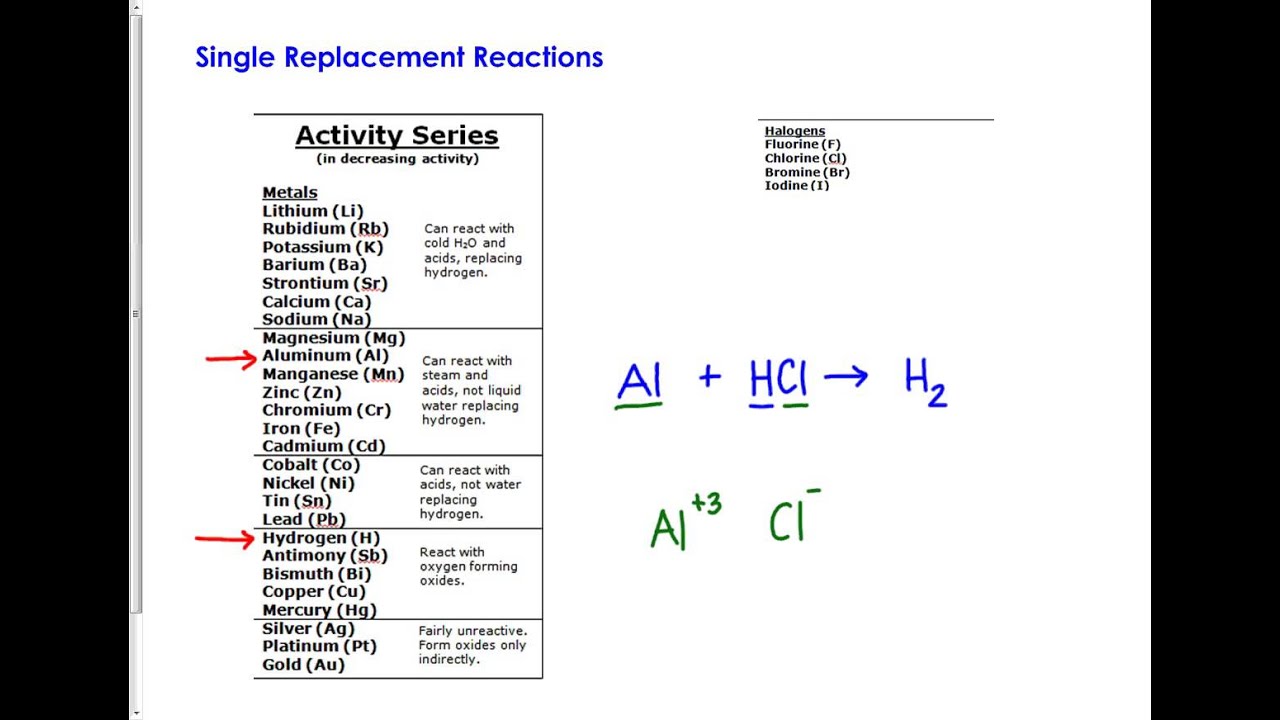
#ACTIVITY SERIES TABLE HOW TO#
Related Byte: Redox Reaction Basics How To Determine Oxidation Number, Oxidizing and Reducing AgentsĪ: Copper because its charge increased from 0 to +2.Ī: H was reduced, making it an oxidizing agent (it oxidized Cu).Ī: Cu was oxidized, making it a reducing agent (it reduced H).Ī: No Since Cu is listed below H in the activity series, it means that hydrogen is actually the stronger reducer. Series Report Historical News Release Tables Maps Calculators Public Data API. Therefore, it makes sense that Mg would be the reducing agent. Place Data in the Table like the one below Solutions Aluminum Nickel Platinum Silver Zinc A. Q: Does this reaction make sense according to the activity series?Ī: Yes Since Mg is listed above H in the activity series, it makes a stronger reducer. Using the activity series table determine if there is a chemical reaction with aluminum, nickel, platinum, silver, or zinc metal reacting with each solution above independently. Activity Series Table Most active (most strongly reducing) metals appear on top, and least active metals appear on the bottom. Activity Series of the Elements Metals Halogens Li React with cold F 2 Rb H 2O and acids. For the reactions that will occur, write the products and balance the equations in the space provided. Write no reactionfor those that will not occur. This is what determines if a reaction will or will not occur.Ī: Magnesium because its charge increased from 0 to +2.Ī: Hydrogen because its charge decreased from +1 to 0.Ī: H was reduced, making it an oxidizing agent (it oxidized Mg).Ī: Mg was oxidized, making it a reducing agent (it reduced H). PART V Using the activity series table, predict whether each of the following reactions will occur. The periodic table reveals a great deal of. The activity series lists elements in their order of strength as oxidizers or reducers. The columns of the periodic table contain elements with similar properties.


 0 kommentar(er)
0 kommentar(er)
